Services
Contract Development and Manufacturing Service
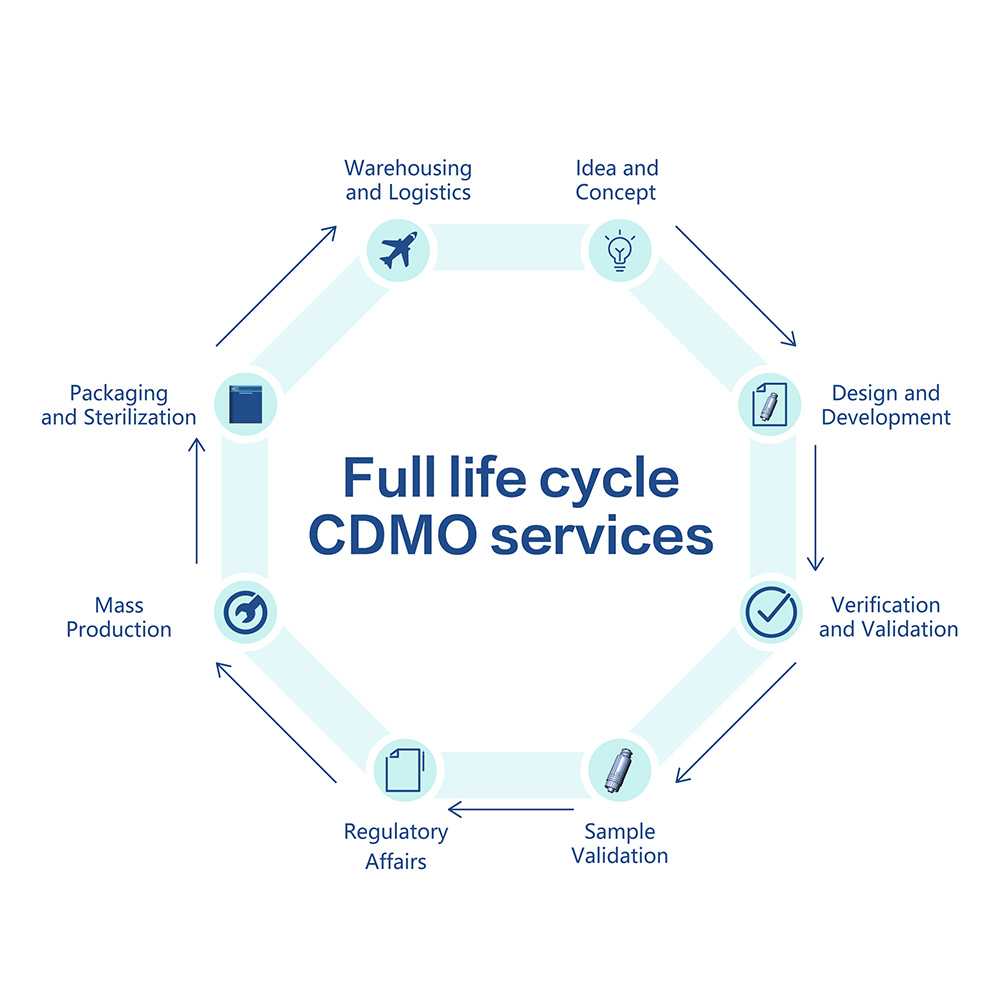
Hantech Medical offers CDMO services for the entire medical product life cycle. Our clients can select the service according to their needs and for all or individual steps of the process: from concept, design and prototyping to finished product regulatory approval, from components mass production to sterilization, as well as warehousing and logistics solutions.
Our production facility is equipped with Class 10,000 (ISO 7) and 100,000 (ISO 8) clean rooms, environment-controlled workshops, full assay testing laboratory, EtO sterilization chambers, and multiple production lines. Hantech Medical is ISO13485 certified, CE certified, NMPA registered, MFDS certified, and FDA registered.